ACRO|Collagen Device Manufacturer for Regenerative Medicine
Tissue Engineering / Biomaterials / Regenerative Medicine


Making the Impossible Possible
ACRO Biomedical's vision is ambitious but clear: to tackle the global organ shortage with collagen scaffolds and organ engineering. Imagine a future where treatment doesn’t depend on donor waiting lists — but is accessible to everyone, everywhere.
Innovation for Vision Restoration
ABCcolla® Ophthalmic Matrix is an innovative solution that helps overcome long-standing challenges in eye surface regeneration.
ABCcolla® Collagen Matrix
BCcolla® Collagen Matrix is a sterile, bioresorbable collagen wound dressing made from porcine collagen using supercritical CO₂ decellularization. It preserves the natural triple-helix collagen structure to support cell proliferation, granulation, and epithelialization.
Key Features:
● Medical-grade collagen wound matrix with high porosity for exudate absorption and moist wound healing
● Flexible, easy to handle, and fully bioabsorbable within 7–14 days — no removal required
● Produced without crosslinking agents, ensuring safety and biocompatibility
Applications:
● Wound care products for chronic and acute wounds
● Collagen dressing for surgical sites, ulcers, and trauma care

ABCcolla® Bone Graft
ABCcolla® Collagen Bone Graft is a decellularized porcine bone graft material prepared using supercritical CO₂ extraction. It is composed of native porcine collagen and bone matrix comparable to the human bone matrix.
Features:
● Designed for filling and augmentation of bone voids or gaps in orthopedic surgeries
● Facilitates cell attachment and supports bone regeneration
● Promotes neovascularization during healing
● Gradually resorbs and is replaced by new bone

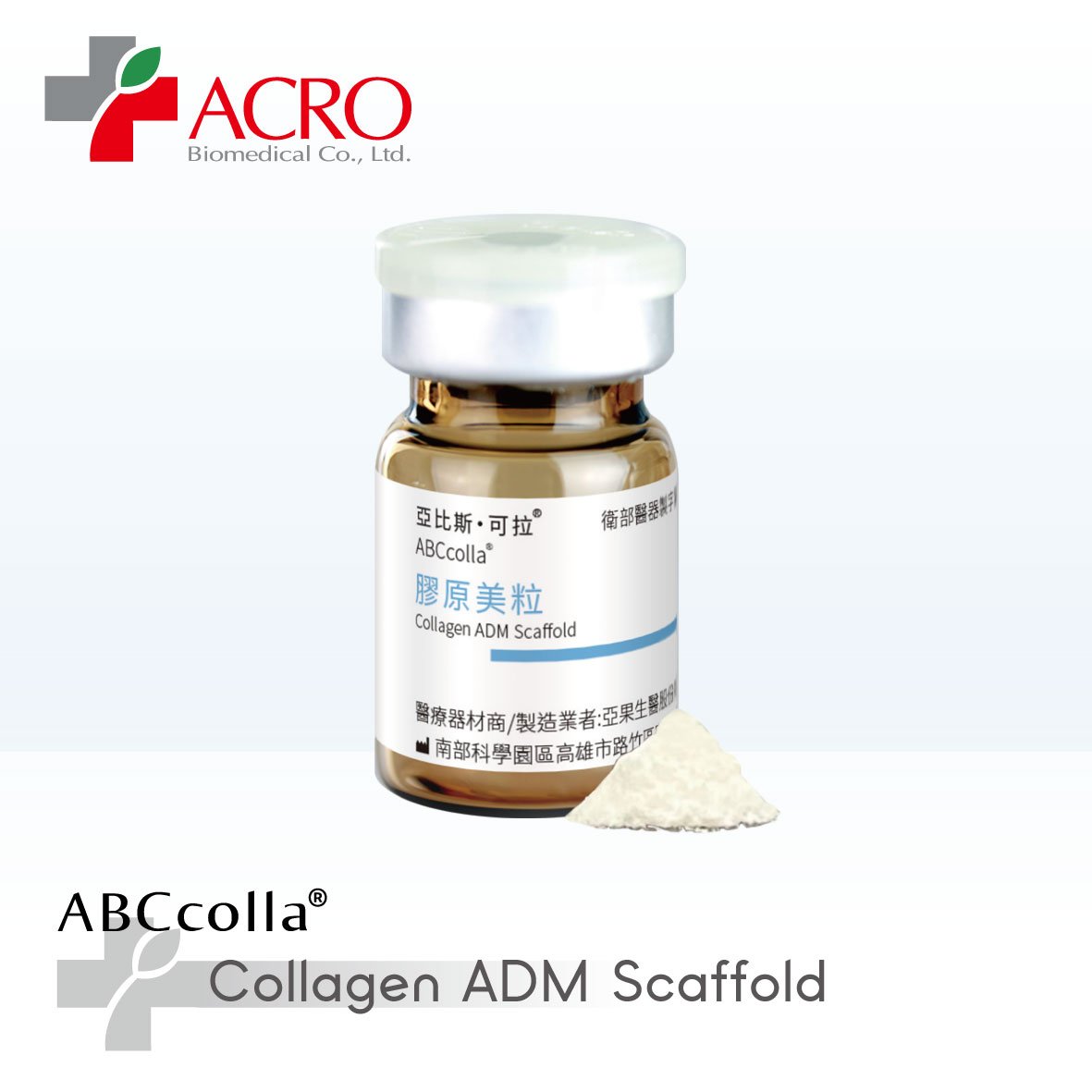
ABCcolla® ADM Scaffold
ABCcolla® ADM Scaffold is a decellularized porcine collagen dermal matrix developed with patented supercritical CO₂ technology.
Features:
● Absorbs wound exudates and maintains a moist healing environment
● Supports tissue regeneration during the healing process
● TFDA- and FDA 510(k)-approved for medical use
● Recipient of the 21st National Innovation Award for its micro-particle ADM application

Get the Perfect Match
Start your 1-on-1 meetings with ACRO to explore new business opportunities.
Trusted Certifications for Quality Assurance
.jpg?width=280&height=280&name=DNV%20ISO13485%20(1).jpg)
Stay Ahead of the Curve!
Subscribe Now for Exclusive Insights, Trends, and Offers Delivered Straight to Your Inbox!

